
NOT FOR HUMAN CONSUMPTION
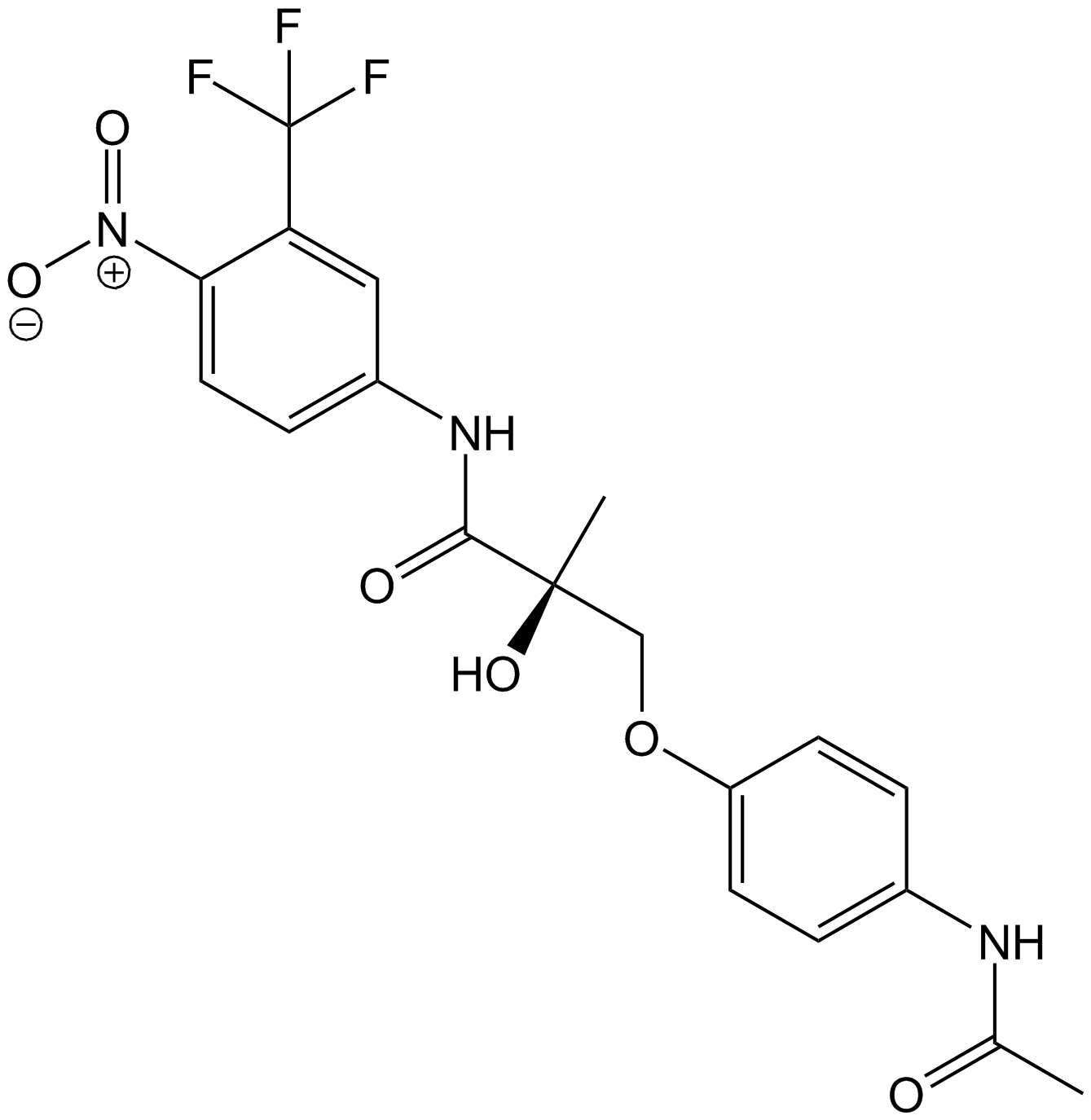
S4(Andarine, GTx-007) is a nonsteroidal selective androgen receptor modulator (SARM) developed by GTx for osteoporosis, muscle wasting, and benign prostatic hyperplasia (BPH). It binds the androgen receptor (AR) with tissue-selective, partial agonist activity—aiming to stimulate bone and muscle while sparing prostate and hair follicles relative to testosterone/DHT. Clinical development never reached approval; most human data are early-phase. It is not approved for any indication and is prohibited by WADA.
Additional Benefits of S-4 Now Under Investigation
| Benefit | Key take-aways |
|---|---|
| 1 Lean mass preservation | In animal and early human studies, S-4 increases lean body mass and muscle strength or preserves them during hypogonadism or caloric deficit, with less virilization than anabolic steroids at comparable anabolic effect. |
| 2 Bone-density support | S-4 improves bone mineral density (BMD) and biomechanical strength in osteopenic/ovariectomized models, mimicking some of testosterone’s skeletal benefits without strong prostate stimulation. |
| 3 Prostate-sparing androgen action | In castrated rodent models, S-4 supports muscle/bone while causing less prostate enlargementthan DHT and even reduces prostate weight under some conditions—basis for its BPH program. |
| 4 Functional performance | Preclinical work shows increased grip strength, running time, and muscle cross-sectional area, indicating true functional gains, not just water retention. |
| 5 Potential cachexia applications | The combination of anabolic and anti-catabolic actions suggests utility for cancer/illness-related cachexia; this remains theoretical and unapproved. |
| 6 Oral delivery | S-4 is orally bioavailable, avoiding injections and allowing once- or twice-daily dosing in trials. |
| 7 Lower androgenic load | Compared to full AR agonists, S-4’s partial agonism and tissue selectivity lead to less virilization, acne, and prostate stimulation at anabolic doses in preclinical work. |
| 8 Limited aromatization | Being nonsteroidal, it does not aromatize to estradiol or reduce 5-α reductase; E2 shifts are secondary (via HPT-axis suppression), not direct conversion. |
| 9 Conceptual stacking with other SARMs | In research, S-4 has been explored as a bone-/cutting-biased SARM alongside more potent anabolic SARMs; this is entirely experimental and not clinically validated. |
2. Molecular Mechanism of Action
2.1 Receptor pharmacodynamics
Target: Andarine binds the androgen receptor (AR) as a partial agonist with a distinct co-activator recruitment profile compared to DHT/testosterone.
Tissue selectivity:
Skeletal muscle & bone: Acts more like an agonist, promoting protein synthesis, antiresorptive signaling, and osteoblastic activity.
Prostate & reproductive tissues: Behaves less strongly than DHT; in some models, functions almost as a functional antagonist by competing with endogenous androgens.
2.2 Down-stream biology
| Pathway | Functional outcome | Context |
|---|---|---|
| AR–ARE transcription | ↑ Myofibrillar protein synthesis, ↓ proteolysis | Skeletal muscle |
| RANKL/OPG balance & osteoblast activity | ↑ BMD, ↑ bone strength | Bone |
| Prostate AR partial agonism | ↓ Prostate weight vs DHT at equal anabolic effect | BPH models |
| HPT-axis feedback | ↓ LH/FSH → ↓ endogenous T with chronic use | Systemic endocrine |
3. Pharmacokinetics (from early studies & preclinical data)
Route: Oral, typically once or twice daily in trials.
Half-life: On the order of hours (short–moderate); supports BID dosing in many experimental protocols.
Absorption: Good oral bioavailability; lipophilic.
Distribution: Binds AR in muscle, bone, prostate, and other AR-rich tissues; crosses into many organs.
Metabolism: Hepatic metabolic clearance; exact CYP profile not publicly characterized.
Excretion: Primarily renal and biliary as metabolites.
4. Pre-clinical & Clinical Evidence
Osteoporosis/Bone models: S-4 restored BMD and bone strength in ovariectomized rats while keeping prostate size relatively low compared with DHT.
Muscle wasting/hypogonadism models: Improved lean mass and strength, outperforming placebo and approaching low-dose testosterone’s anabolic effect with less androgenic tissue impact.
BPH/androgen-deprivation settings: Showed ability to maintain muscle/bone under androgen deprivation while not excessively stimulating prostate, making it attractive in theory as an adjunct to androgen-deprivation therapy.
Evidence quality note: Most data are animal studies plus small, early-phase human trials. There are no large Phase 3 data, no approved indications, and the full risk–benefit profile at therapeutic doses remains incompletely defined.
5. Emerging Clinical Interests (conceptual)
| Field | Rationale | Status |
|---|---|---|
| Male osteoporosis / osteopenia | Bone-anabolic + prostate-sparing | Preclinical/early clinical |
| Sarcopenia / frailty | Muscle maintenance with lower androgenic load | Concept |
| Cachexia (cancer/HIV/CHF) | Anticatabolic, oral, anabolic | Preclinical concept |
| Adjunct in androgen-deprivation (prostate CA) | Preserve muscle/bone, not prostate | Concept; safety complex |
| Female osteoporosis (post-menopausal) | Bone support without virilization | Animal data only |
6. Safety and Tolerability
Important: Much of the human safety profile is inferred from limited trial data and off-label/grey-market use, not long, high-quality RCTs.
6.1 Commonly reported / expected
Visual disturbances (hallmark of S-4):
Yellow tint to vision or altered color perception.
Night-vision difficulty (reduced adaptation in low light).
Usually dose-related and reversible after discontinuation in reports; likely due to S-4 or its metabolites affecting retinal signaling (exact mechanism uncertain).
Endocrine suppression:
Decreased LH/FSH, total and free testosterone with prolonged exposure.
Potential testicular atrophy and transient subfertility with chronic use.
Androgenic/skin: Mild acne, oily skin, hair shedding in susceptible individuals—generally milder than full androgenic steroids at equivalent anabolic effect.
Lipids: HDL reduction and possible LDL increase, as with many androgens/SARMs.
Liver: Mild ALT/AST elevations reported anecdotally; robust hepatotoxicity data are lacking but caution is appropriate.
6.2 Less common / theoretical
Cardiometabolic: Possible BP increases, hematocrit drift, and unknown long-term cardiovascular risk.
Mood/neurologic: Some users report irritability, decreased libido with endogenous suppression; data are anecdotal.
Reproductive: Potential impact on spermatogenesis and fertility with long-term use; recovery time after cessation is not well characterized.
Oncology: Long-term tumor risk with chronic partial AR stimulation/inhibition has not been mapped; caution in anyone with a history of hormone-sensitive cancers.
6.3 Anti-doping / legal status
WADA: SARMs (including S-4/Andarine) are prohibited at all times (S1.2 anabolic agents).
Regulation: S-4 is not an approved medication; many jurisdictions treat SARMs sold for human consumption as unlawful or controlled. Grey-market powders/capsules often have mislabeling and contamination.
7. Comparative Snapshot (SARMs & anabolics)
| Feature | S-4 (Andarine) | LGD-4033 (Ligandrol) | RAD-140 (Testolone) | Testosterone (TRT) |
|---|---|---|---|---|
| AR profile | Partial agonist, tissue-selective | Potent AR agonist | Potent, somewhat selective | Full agonist |
| Bone focus | Strong | Strong | Moderate | Strong |
| Prostate stimulation | Lower; sometimes ↓ | Moderate | Moderate | Higher |
| Vision AEs | Characteristic (night/yellow) | None typical | None typical | None |
| HPT suppression | Yes (moderate) | Yes (strong) | Yes (strong) | Yes |
| Legal/approval | Unapproved, SARM ban | Unapproved, SARM ban | Unapproved, SARM ban | Approved (TRT) |
8. Regulatory Landscape
No approvals in US/EU/UK/most jurisdictions.
Used only in research; any therapeutic development has stalled in favor of newer SARMs or different anabolic approaches.
Widely sold online as a research chemical, often in non-GMP conditions with unverified purity and dose.
9. Practical Take & Future Directions
For now: S-4 is best regarded as a historical SARM prototype and research tool, not a clinical therapy.
Main unique point: Its bone-/muscle-anabolic + prostate-sparing profile and distinct visual side effects make it mechanistically interesting but clinically problematic.
Research priorities:
Detailed characterization of the ocular mechanism and long-term retinal safety.
Development of next-generation SARMs that retain S-4’s bone/muscle selectivity without vision issues.
More complete mapping of lipid, cardiovascular, and reproductive impacts of AR partial agonism vs full agonism.





Beoordelingen
Er zijn nog geen beoordelingen.