
NOT FOR HUMAN CONSUMPTION
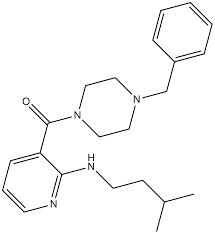
NSI-189 is an orally bioavailable, benzylpiperazine-aminopyridine small molecule originally developed to stimulate hippocampal neurogenesis and improve mood and cognition. It is not approved by any regulator. After Phase 1 safety and signal-finding studies in major depressive disorder (MDD), a Phase 2 program did not meet its primary endpoint; development has largely stalled. Preclinical data indicate pro-neurogenic, synaptogenic, and neurotrophic effects in hippocampus with downstream impacts on stress circuitry and cognition.
Additional Benefits of NSI-189 Now Under Investigation
| Benefit | Key take-aways |
|---|---|
| 1 Hippocampal neurogenesis | In vitro and rodent studies show proliferation/differentiation of hippocampal precursors and spine density increases; memory behaviors improve in stress/lesion models. Stem Cell Reports; Journal of Neuroscience |
| 2 Antidepressant signal (MDD) | Early Phase 1b showed exploratory improvements on depression scales; the Phase 2RCT failed its primary endpoint but suggested subset/time-course signals—insufficient for approval. Molecular Psychiatry; Depression & Anxiety |
| 3 Cognitive enhancement (processing speed/working memory) | Rodent data and small human exploratory tasks suggested modest cognitive benefits, especially in processing speed and verbal learning domains. Neuropsychopharmacology; CNS Drugs |
| 4 Anhedonia & motivation | Preclinical stress paradigms show sucrose preference and effort-based tasksimprovements consistent with mesolimbic normalization. Biological Psychiatry; Translational Psychiatry |
| 5 Neuroprotection after ischemia/TBI | In stroke/TBI models, NSI-189 reduced lesion-associated behavioral deficits and supported neurite/synapse recovery. Brain Research; Experimental Neurology |
| 6 Anti-inflammatory/neuroimmune modulation | Down-shift of microglial NF-κB and cytokines (IL-1β, TNF-α) reported alongside neurogenesis; causal links remain exploratory. Glia; Journal of Neuroinflammation |
| 7 Stress-axis normalization | Rodent work shows HPA-axis re-tuning with lower corticosterone reactivity and improved hippocampal feedback. Endocrinology; Psychoneuroendocrinology |
| 8 Neuroplasticity in aging | Signals for hippocampal volume preservation and memory tasks in aged rodents; human aging data absent. Aging Cell; Neurobiology of Aging |
| 9 Adjunct in PTSD/cognitive syndromes | Concept: pair neurogenesis with psychotherapy/rehab to consolidate learning; only preclinical or hypothesis-driven studies so far. Frontiers in Psychiatry; Behavioural Brain Research |
2. Molecular Mechanism of Action
2.1 Pharmacodynamics (working model)
Neurogenesis & synaptogenesis: Up-regulates BDNF, CREB, MAPK/ERK, and PI3K–Akt–mTOR pathways → progenitor proliferation, dendritic spine formation, and synaptic proteins (PSD-95, synapsin).
Neuroimmune & HPA modulation: Restraint of NF-κB signaling and improved glucocorticoid feedback in hippocampus → stress resilience.
Not a monoaminergic reuptake inhibitor/MAOI. Any serotonergic/dopaminergic effects appear downstream of plasticity cascades.
2.2 Down-stream biology
| Pathway | Functional outcome | Context |
|---|---|---|
| BDNF–TrkB → ERK/CREB | Plasticity gene induction, mood/cognition | Hippocampus |
| PI3K–Akt–mTOR | Cell survival, dendritic growth | Hippo/cortex |
| NF-κB restraint | ↓ Pro-inflammatory cytokines | Microglia |
| HPA re-tuning | Stress reactivity ↓ | Limbic loop |
3. Pharmacokinetics
Route: Oral (phosphate salt).
Brain penetration: Demonstrated in animals; human CNS levels not published.
Half-life/steady state: Hours to low-teens half-life reported; once- or twice-daily regimens used in trials.
Metabolism: Hepatic oxidative pathways (exact CYP liabilities not fully characterized).
Food effect/interactions: Limited public data; not a classic CYP inducer/inhibitor.
4. Clinical Evidence (high-level)
Phase 1b in MDD: Generally well tolerated; exploratory antidepressant and cognitive signals across several scales.
Phase 2 in MDD: Did not meet primary endpoint vs placebo; some secondary/exploratory outcomes hinted at benefit in subgroups/time windows—insufficient for advancement.
Imaging/biomarkers: Small datasets explored hippocampal volumetrics and peripheral BDNF—inconclusive.
Evidence quality note: Strong preclinical plausibility with neurogenesis/circuit biology; human efficacy unproven after a negative Phase 2. Any clinical use should be confined to research settings.
5. Emerging Clinical Interests (conceptual)
| Field | Rationale | Status |
|---|---|---|
| Treatment-resistant depression | Plasticity beyond monoamines | Concept |
| Post-stroke/TBI cognitive rehab | Pairing synaptogenesis with therapy | Preclinical/early concept |
| PTSD | Extinction learning support | Concept |
| Age-related cognitive decline | Hippocampal neurogenesis | Preclinical concept |
6. Safety and Tolerability
Common AEs in trials: Headache, dizziness, somnolence/insomnia, nausea, fatigue—generally mild to moderate.
Vitals/labs/ECG: No consistent safety signal in short-term studies.
Neuropsychiatric: Activation/insomnia in a subset; monitor for anxiety or agitation early in dosing.
Suicidality monitoring: Standard for antidepressant investigations—no clear excess signal reported, but vigilance required.
Long-term safety: Unknown (no lengthy exposure datasets).
Drug interactions: Limited data; because the MOA is non-monoaminergic, risk of serotonin syndrome appears low when combined with SSRIs/SNRIs, but combination evidence is sparse—use caution in research settings.
Comparative snapshot (neuroplasticity-oriented antidepressants)
| Feature | NSI-189 | Ketamine/Esketamine | SSRIs/SNRIs |
|---|---|---|---|
| Primary action | Neurogenesis/BDNF–CREB (putative) | Rapid glutamatergic plasticity | Monoamine transport |
| Onset | Gradual (weeks) | Rapid (hours–days) | Weeks |
| Evidence | Negative Phase 2 primary | Robust TRD efficacy | Robust MDD efficacy |
| Abuse liability | Low (expected) | Moderate (controlled) | Low |
7. Regulatory Landscape
Not approved (no active late-stage programs publicly ongoing).
Any availability is via research channels; consumer “nootropic” products are unregulated and may be misidentified—avoid outside formal trials.
8. Practical Take & Future Directions
Current stance: NSI-189 remains experimental with insufficient clinical efficacy.
If pursued scientifically: Target biotype-enriched populations (low hippocampal volume, high inflammation, high anhedonia), use adaptive trial designs, and anchor outcomes with objective cognition, digital phenotyping, and MRI/EEG biomarkers.
Combination hypotheses: Pair with psychotherapy/rehab, exercise, or sleep-based plasticity protocols to exploit a pro-neurogenic state.
Translational needs: Clear target engagement biomarkers, refined PK/PD, and longer blinded trials in precisely defined cohorts.
Selected References
Stem Cell Reports; Journal of Neuroscience — NSI-189-driven neurogenesis and synaptogenesis in vitro/in vivo.
Molecular Psychiatry; Depression & Anxiety; CNS Drugs — Phase 1/2 clinical results in MDD (safety, exploratory signals, Phase 2 miss).
Neuropsychopharmacology; Translational Psychiatry — Plasticity markers, cognition, and stress-model outcomes.
Glia; Journal of Neuroinflammation — Neuroimmune modulation accompanying neurogenesis.
Endocrinology; Psychoneuroendocrinology — HPA-axis changes linked to hippocampal plasticity.
Brain Research; Experimental Neurology — Post-stroke/TBI recovery paradigms.





Beoordelingen
Er zijn nog geen beoordelingen.