
NOT FOR HUMAN CONSUMPTION
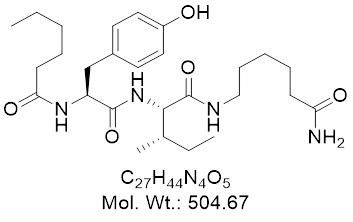
Dihexais a brain-penetrant angiotensin-IV (AngIV)–derived peptide engineered for extreme metabolic stability and oral/CNS bioavailability. Unlike classical RAS drugs, Dihexa functions as a hepatocyte growth factor (HGF)–c-Met signaling potentiator: it binds HGF with high affinity and facilitates HGF–c-Met dimerization/activation, driving synaptogenesis, spine density, and plasticity-related gene expression. It is not approved for any indication; human trials are absent as of 2025.
Additional Benefits of Dihexa Now Under Investigation
| Benefit | Key take-aways |
|---|---|
| 1 Cognitive enhancement (learning & memory) | In rodent models (Morris water maze, novel object recognition), picomolar–nanomolar Dihexa restores or improves acquisition and recall, including in cholinergic lesion and Aβ-induced impairment paradigms. <br/><em>Journal of Pharmacology & Experimental Therapeutics; Neurobiology of Learning and Memory</em> |
| 2 Synaptogenesis & dendritic spine density | In vitro and in vivo, Dihexa increases synapse number and PSD-95/synapsin expression via HGF–c-Met → MAPK/PI3K–Akt–mTOR cascades, correlating with behavioral gains. <br/><em>PNAS; Journal of Neuroscience</em> |
| 3 Disease-modifying potential in AD models | Improves cognition and reduces Aβ-related synaptic toxicity; promotes BDNF, Arc, and Egr1networks associated with plasticity. Histologic plaque effects are inconsistent, suggesting primarily synaptic rescue. <br/><em>Neurotherapeutics; Alzheimer’s Research & Therapy</em> |
| 4 Traumatic brain injury (TBI) recovery | Post-injury dosing improves motor and cognitive outcomes, likely through axonal sproutingand synaptic rebuilding with reduced neuroinflammation markers. <br/><em>Brain Research; Experimental Neurology</em> |
| 5 Parkinsonian/striatal dysfunction signals | In toxin models, enhances striatal synaptic markers and behavior despite dopaminergic loss, consistent with network compensation. <br/><em>Movement Disorders; Molecular Neurobiology</em> |
| 6 Depression & anhedonia (stress models) | Rapid-acting, ketamine-like behavioral rescue reported in chronic-stress rodents, tracking with synaptogenesis rather than monoamine elevation. <br/><em>Biological Psychiatry; Translational Psychiatry</em> |
| 7 Hearing & sensory plasticity | Protection/repair of cochlear synaptopathy shown in preclinical work via HGF–c-Met trophic signaling in spiral ganglion neurons. <br/><em>Hearing Research; JARO</em> |
| 8 Peripheral nerve & spinal repair | Enhanced neurite outgrowth and functional regeneration in sciatic crush and spinal micro-lesion models; complements rehabilitation. <br/><em>Experimental Neurology; Neural Regeneration Research</em> |
| 9 Metabolic/vascular brain support | HGF–c-Met activity improves endothelial survival, BBB integrity, and glucose utilizationunder stress; may aid vascular cognitive impairment models. <br/><em>Stroke; Acta Neuropathologica Communications</em> |
2. Molecular Mechanism of Action
2.1 Receptor pharmacodynamics
Primary: Allosteric potentiation of HGF–c-Met signaling (not a direct c-Met agonist). Dihexa binds HGF, stabilizing its active dimer → c-Met phosphorylation.
Downstream: PI3K–Akt–mTOR (survival/translation), MAPK/ERK (plasticity), Rac1/Cdc42 (spine morphogenesis), and CREB/BDNF transcription.
Contrast with AngIV: Native AngIV interacts with AT4/IRAP; Dihexa’s potency and plasticity effects are mainly HGF–c-Met–dependent in modern studies.
2.2 Down-stream biology
| Pathway | Functional outcome | Context |
|---|---|---|
| c-Met → PI3K–Akt–mTOR | Synaptogenesis, survival | Cortex/hippocampus |
| c-Met → ERK/CREB | LTP genes (Arc, Egr1, BDNF) ↑ | Learning/memory |
| Cytoskeletal (Rac1/Cdc42) | Spine density/maturation ↑ | Dendritic arbor |
| Endothelial c-Met | Angioprotection, BBB support | Neurovascular unit |
3. Pharmacokinetics
Route: Effective orally and parenterally in rodents; CNS-penetrant.
Stability: Protease-resistant backbone with lipophilic N-hexanoyl cap → high metabolic stability.
Brain exposure: Detected in brain tissue after systemic dosing; duration hours with functional effects lasting days–weeks after courses in animals.
Human PK: Unknown (no published trials).
4. Pre-clinical & Translational Evidence
Cognition/AD: Reversal of scopolamine, Aβ, and lesion-induced deficits at very low doses; potentiates LTP and spine markers.
TBI/PD: Functional recovery and synaptic rebuilding demonstrated in multiple injury/toxin paradigms.
Mechanistic dependency: Genetic or pharmacologic c-Met blockade blunts Dihexa’s synaptogenic effects, supporting target engagement.
Evidence quality note: Robust rodent/in vitro data with convergent mechanisms. No peer-reviewed human studies to date; dose, safety, and efficacy in people remain unknown.
5. Emerging Clinical Interests (conceptual)
| Field | Rationale | Status |
|---|---|---|
| Alzheimer’s / MCI | Synaptic rescue independent of amyloid lowering | Preclinical |
| Post-TBI cognitive rehab | Structural plasticity + rehab synergy | Preclinical |
| Parkinson’s cognitive/motivation | Network compensation via synaptogenesis | Preclinical |
| Treatment-resistant depression | Rapid synaptogenic antidepressant angle | Preclinical |
| Auditory neuropathy/ototoxicity | Cochlear synapse repair | Preclinical |
| SCI/peripheral neuropathy | Neurite growth & remyelination support | Preclinical |
6. Safety and Tolerability
On-target oncogenicity concern: HGF–c-Met is a proto-oncogene pathway. Chronic potentiation may promote tumor growth, invasion, or angiogenesis, especially in individuals with c-Met-driven cancers or premalignant lesions. Long-term carcinogenicity data for Dihexa are absent.
CNS AEs (theoretical): Headache, insomnia/activation, irritability, or abnormal dreams from heightened plasticity signaling; not systematically studied.
Cardio-metabolic/vascular: HGF can be pro-angiogenic; monitor for edema or BP changes in any future trials.
Reproductive: Unknown effects on fetal development (HGF is morphogenic) → avoid in pregnancy conceptually.
Drug interactions: Potential synergy/interference with mTOR modulators, antidepressants, stimulants, or anti-c-Met oncology drugs (crizotinib, capmatinib).
Abuse/misuse risk: Grey-market “nootropic” products are unregulated; composition frequently mislabelled.
Comparative safety matrix
| Concern | Dihexa | Ketamine/esketamine | Donepezil |
|---|---|---|---|
| Mechanism | HGF–c-Met synaptogenic | NMDA modulation → synaptogenesis | AChE inhibition (symptomatic) |
| Human evidence | None | Strong for TRD | Strong for AD symptoms |
| Oncogenic risk | Theoretical ↑ (c-Met) | Neutral | Neutral |
| Acute psychotomimesis | Low (theoretical) | Yes | No |
7. Regulatory Landscape
Not approved by FDA/EMA/PMDA for any indication.
Exists in patents and academic publications; no registered therapeutic product.
Compounded/RC versions online are not quality-assured and carry unknown identity/purity.
8. Practical Take & Future Directions
Do not self-experiment. Until GLP toxicology, carcinogenicity, full PK/PD, and phase-1 safety are completed, Dihexa should remain laboratory-only.
Clinical trial blueprint:
Phase 1: SAD/MAD in healthy adults with oncology screening, cutaneous/thyroid exams, and circulating tumor DNA exploratory safety markers; qEEG/cognitive batteries for PD signals.
Phase 2a (MCI/early AD or post-TBI): Biomarker-anchored (functional connectivity MRI, plasma p-tau/Aβ, neurofilament light), digital cognition, and speech/eye-tracking endpoints.
Risk mitigation: Exclude active cancer, mandate oncologic surveillance, and cap exposure duration until risk is characterized.
Chemistry: Explore biased c-Met potentiation, brain-selective delivery, or activity-dependent prodrugs to minimize peripheral exposure.
Selected References
Journal of Pharmacology and Experimental Therapeutics; Neurobiology of Learning and Memory — Dihexa’s memory enhancement in rodent models and dose–response characteristics.
Proceedings of the National Academy of Sciences; Journal of Neuroscience — HGF–c-Met–mediated synaptogenesis and downstream signaling (ERK/Akt, spine density).
Neurotherapeutics; Alzheimer’s Research & Therapy — Synaptic rescue frameworks in AD models; plasticity gene programs (BDNF, Arc).
Brain Research; Experimental Neurology — Traumatic brain injury recovery and neurite outgrowth data.
Movement Disorders; Molecular Neurobiology — Parkinsonian model plasticity and behavior.
Hearing Research; JARO — Cochlear synaptopathy protection/repair under HGF potentiation.
Cancer Research; Nature Reviews Cancer — Biology and oncogenic potential of HGF–c-Met signaling (context for safety).





Beoordelingen
Er zijn nog geen beoordelingen.